Research Vision: Towards Very Early Identification and Intervention
The first thousand days of life between conception and age two is a crucial period of heightened plasticity, where multiple organ systems (e.g. immune, lung, brain) are functionally immature and are highly sensitive to the environment. Adverse exposures (e.g. maternal stress, poor nutrition, severe infections, antibiotics, toxins, pollutants) during this early window of susceptibility can result in the programming of potentially life-long susceptibility to chronic diseases. Notably, the prevalence of complex diseases has increased dramatically over the last few decades, which is thought to be due to a mismatch between evolved human physiology and rapid changes in our environment, diet, and lifestyle. The cost of new medications is also rising, placing an increased financial burden on families and healthcare systems. To address this issue, my vision is to develop the tools, which will enable very early identification of at-risk individuals, combined with targeted interventions to promote healthy developmental trajectories, and avoid disease transitions.
In utero, key effector functions of the immune system exemplified by interferon production are attenuated because they are toxic to the placenta, and in the early postnatal period, capacity to produce interferons at birth is inversely related to risk for severe lower respiratory infections and the subsequent development of asthma. During the first seven days of life, a series of rapid and dynamic changes are initiated within the innate immune system, which trigger upregulation of host defence functions. However, susceptibility to infections in general remains heightened throughout infancy, and in particular severe lower respiratory infections are the most frequent cause of hospitalisation in this age group. It is now recognised that exposure to microbial stimuli in utero and early infancy is essential for driving immune development, and the kinetics of the final stages of this process during the early postnatal period is a major determinant of 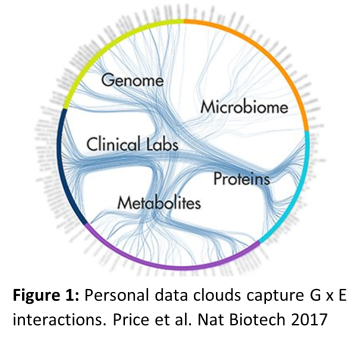 susceptibility versus resistance to infections, allergies, and asthma. The gut microbiota is an essential source of microbial signals to stimulate immune maturation; however, neonatal colonization is a delicate and dynamic process that is highly sensitive to the environment (e.g. mode of birth, breast feeding, antibiotics). Therefore, understanding the factors that determine risk for immune-mediated diseases throughout the life-course will require an integrated perspective of the complex and dynamic interactions between environmental exposures, the developing immune system, the gut microbiome, and the affected organ system. The long-term goal of my work is to develop the tools to generate personal data clouds for infants and children (Figure 1), which track developmental trajectories, predict disease transition points, and unveil actionable targets for disease treatment and/or prevention.
susceptibility versus resistance to infections, allergies, and asthma. The gut microbiota is an essential source of microbial signals to stimulate immune maturation; however, neonatal colonization is a delicate and dynamic process that is highly sensitive to the environment (e.g. mode of birth, breast feeding, antibiotics). Therefore, understanding the factors that determine risk for immune-mediated diseases throughout the life-course will require an integrated perspective of the complex and dynamic interactions between environmental exposures, the developing immune system, the gut microbiome, and the affected organ system. The long-term goal of my work is to develop the tools to generate personal data clouds for infants and children (Figure 1), which track developmental trajectories, predict disease transition points, and unveil actionable targets for disease treatment and/or prevention.


