Inflammation provides a fundamental role in directing the innate immune response against invading microorganisms, promoting adaptive immunity and initiating wound healing following resolution of infection and injury. These processes involve the coordinate activation of multiple cell types and production of secreted factors that are tightly regulated by cell signal transduction pathways. While great emphasis has been placed on how these events promote acute inflammation to combat infection, the dysregulation of these signaling networks and aberrant immune activation can lead to chronic inflammatory disorders through not fully understood mechanisms. Moreover, chronic inflammation is highly associated with multiple forms of cancer, with the link between inflammatory bowel disease and colon cancer representing a prime example of this relationship.
Inflammatory responses are initiated upon activation of pattern recognition receptors (PRRs) that are largely expressed by innate immune cells as well as epithelial cells. These PRRs include cell surface-expressing Toll-like receptors (TLRs) and cytosolic nucleotide-binding, leucine-rich repeat (or NOD-like receptor, NLR) proteins, which recognize conserved microbial components (e.g., LPS and flagellin) or host-derived factors released during cellular injury and death (e.g., extracellular ATP). This large repertoire of sensors allows the innate immune system to react to a diverse range of infectious organisms and cellular insults. While the majority of PRRs promote inflammation, a subset of these proteins perform important anti-inflammatory roles (e.g., NLPR12 and NLRC3) and/or other distinct functions.
One example of dual-functioning PRR is the cytosolic double-stranded DNA sensor Absent in Melanoma 2 (AIM2). DNA binding causes AIM2 to recruit the adapter protein ASC and the pro-form of the enzyme Caspase-1, leading to formation of the multiprotein complex termed the inflammasome. This protein oligomerization event leads to caspase-1 activation and subsequent production of the active pro-inflammatory cytokines IL-1b and IL-18 (see figure), which play an important role in host defense against double stranded DNA viruses and intracellular bacteria. My previous work identified an inflammasome-independent function for AIM2 in suppressing tumorigenesis by acting as a negative regulator of the PI3K/Akt signaling pathway of cellular survival in intestinal epithelial cells, which limits tumorigenesis in multiple models of colon cancer. Previous clinical studies have linked the loss of AIM2 to late-stage metastatic colon cancer and reduced patient survival, suggesting targeting Akt as a personalized therapy for individuals with AIM2 low/null-expressing tumors.
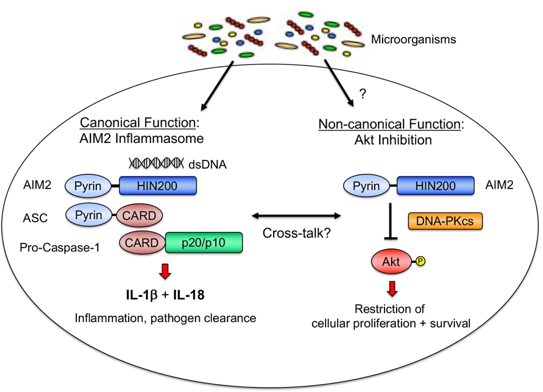
Major topics that will be addressed by my research group include: 1) How dsDNA influences the non-canonical function of AIM2; 2) Understanding the relative contribution and potential cross-talk between the canonical and non-canonical functions of AIM2 in both innate immune cells and epithelial cells; and 3) Elucidating the role of AIM2 during other forms of cancer and chronic inflammatory disorders, with a focus on translational research.

